|
| 产品描述 |
| Canagliflozin (INN, trade name Invokana) is a drug for the treatment of type 2 diabetes. It was developed by Mitsubishi Tanabe Pharma and is marketed under license by Janssen, a division of Johnson & Johnson. Canagliflozin is an inhibitor of subtype 2 sodium-glucose transport protein (SGLT2), which is responsible for at least 90% of the glucose reabsorption in the kidney. Blocking this transporter causes blood glucose to be eliminated through the urine. In March 2013, canagliflozin became the first SGLT2 inhibitor to be approved in the United States. |
| 化学结构 |
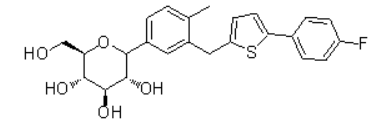 |
| 化学名称 |
| (3R,4R,5S,6R)-2-(3-((5-(4-fluorophenyl)thiophen-2-yl)methyl)-4-methylphenyl)-6-(hydroxymethyl)tetrahydro-2H-pyran-3,4,5-triol |
| SMILES Code |
| O[C@H]1C(C2=CC=C(C)C(CC3=CC=C(C4=CC=C(F)C=C4)S3)=C2)O[C@H](CO)[C@@H](O)[C@@H]1O |
| 基本信息 |
产品编号:XM112
产品名称:Canagliflozin
别名:JNJ 24831754; INJ24831754; JNJ-24831754; JNJ24831754AAA; JNJ24831754ZAE; TA 7284; TA-7284; TA7284; Canagliflozin; Trade name: Invokana.
CAS#:842133-18-0
分子式:C24H25FO5S
精确分子量:444.14067 2
分子量:444.52
|
| 物化性质 |
外观: 白色固体粉末
纯度: >98%
运输信息: 非危险化学品,适于常温或冰袋运输。
储存条件: 低温,干燥,避光。
溶解性: 溶于DMSO, 不溶于水
储存期限:3年 -20℃固体储存
2 年 -80℃ 溶液储存 |
该产品只供科研使用,不能给病人提供。
|
|
|
